
|
FDA GRANDFATHER Clause Petition |

|
|---|
FDA
GRANDFATHER CLAUSE PETITION
FORWARD:
No one here is kidding themselves, this petition (introduced by a museum with [how’s that song go] “NO lawyers, gun’s or money”) is going no-where and hasn’t a prayer. If it was a simple matter of pointing out the obvious facts, or arguing the law, FDA Grandfather approval of Medical Cannabis would be a shoe-in. But, we all know the reality of the matter. The political and economic forces aligned against us are simply too powerful --- We stand no chance.
However, it has been noticed that every time the Narc’s (and their fellow travelers), are forced to respond to such petitions, legal challenges etc. . . they sort of always end up making fools of themselves -- Exposing their true motives to yet more of the public.
Plus this museum curator must pay back a debt that he personally owes to the late Dr. Tod Mikuriya. In effect, I promised him that I/we would at least make the attempt.
[Warning: -- It is possible that a few transcriber errors have been made, with reference to the actual petition filed with the FDA. Hopefully they have been kept to a minimum.
TO: DOCKETS MANAGEMENT BRANCH
Food and Drug Administration
Department of Health and Human Services
12420 Parklawn Drive, Room 1-23
Rockville, MD 20857
PETITION and NOTICE OF EXEMPTION
The undersigned, William McPike Esquire and Beverly Mikuriya, M.D. submit this petition and notice of exemption under 21 U.S.C. § 321(p) to request the Commissioner of Food and Drugs to issue a ruling that the products listed below are exempt from all of the new drug provisions of the act under the exemption for products marketed before June 25, 1938 (more commonly known as the "grand- father clause"). 21 CFR § 314.200(e)(2)
PRODUCTS SUBJECT TO EXEMPTION
-
(1)- BULK CANNABIS (in either Pressed, Loose Leaf, Sifted, Grounded, or Powdered forms). -- See Addendum F for exact definition.
(2)- CANNABIS in its TINCTURE or Liquid form; (A mixture of Alcohol and Cannabis); manufactured as per Lloyd Brothers Corp., or “Eclectic” Medical Standards.”
FORMULATIONS, USES, LABELING, and MARKETING:
Attached hereto are copies of pertinent documents and records that establish the formulations, the uses, the labeling, and the marketing of the above identified products at the time of the initial marketing of those products. These documents and/or records are best summarized as follows:
LLOYD BROTHERS, CANNABIS TINCTURE:
Documentation ---Establishing the edibility of Cannabis Tincture (as manufactured per Lloyd Brothers Corp., or “Eclectic” Medical standards), for Grandfathering:
Documentation dealing with its marketing time line:
- Addendum - M: Contains documentation establishing that the product in question was being marketed and sold between Jan 1, 1907 when the Pure Food and Drug Act took effect, and June 25, 1938 when the Pure Food, Drug and Cosmetics Act took effect.
- Addendum - H: Contains documentation establishing that the product in question was being marketed and sold on and after June 25, 1938 when the Pure Food, Drug and Cosmetics Act took effect.
- Addendum - E: Contains Federal Tax Records dealing with the MTA (Marihuana Tax Act) which took effect on Oct 1937. This documentation along with those found in Addendum - H: establish that last Marketing date as being Dec 31, 1945 as follows:

Also from Addendum - H, we see selected pages from their 1941 price & product catalog showing that Lloyd Brothers was still marketing their Cannabis Tincture at that time. Addendum - E lists the number of manufacturers [look under Ohio] that paid the MTA for the given years. Note that there is only one listed Ohio manufacturer [Lloyd Brothers] who paid the MTA during the years 1937 - through 1944. But that no one was paying the MTA after Jan 1, 1946.
The tax records prove that this cannabis product was being sold and marketed between the years Jan 1, 1870 and until Dec 31, 1945. Clearly, this product qualifies for exemption under the cited regulations.
FACTORS DEALING WITH FORMULATION OF CANNABIS TINCTURE:
TINCTURE CANNABIS [Lloyd Brothers Co.]
Attached hereto are true and correct copies of pertinent documents and records maintained by the manufacturer/distributor, or other sources, that establish the exact manufacturing Procedures/Formulation used by the Lloyd Brothers Corporation from the product’s inception [Jan 1, 1870] until marketing ceased on Jan 1, 1946. These documents and/or records can best be summarized as follows:
Like ALL other tinctures, Lloyd’s “Specific Medicine” Cannabis tincture is nothing more than a liquefied form of Medical Cannabis. And like every other tincture manufacturer, it faced the same general problem. The problem being, that the active ingredient of Cannabis is an OIL-based substance and the simple fact that oil and water don’t mix, but that oil and alcohol do. Which is why the ALL Cannabis tinctures are nothing more than a mixture of the [oily active ingredient of] the flowering tops of the Hemp/Cannabis sativa l. plant and Alcohol. That’s the entire formula, comprised of only these two ingredients.The following diagram [taken from a popular science magazine] is offered for example and describes the Lloyd Brother’s basic distilling process, with Belladonna used in this example.
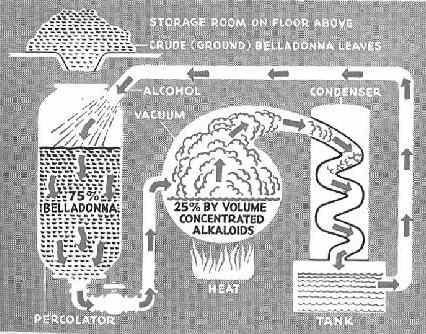
Note that you start by placing the plant matter in one tank [substitute the word Cannabis for the word Belladonna in the diagram above] and allow the alcohol to extract the oily [active ingredient] from the plant matter. In this case, the formula will be 25% Cannabis extract and 75% Alcohol. However as is obvious, the exact percentages will vary depending upon the amount of active ingredient that was originally in the plant matter. And this is key to understanding the very existence of the eclectic [A] medical standard.
- Addendum - J: Contains documentation establishing the U.S. patent (No. 777,115) of the actual cold fire still used to manufacture the Lloyd Brothers tincture throughout the required period for FDA grandfathering.
“The trash on the market, under the free-to-all name of ‘fluid extracts,’ illustrates that, for protection, our preparations [referring to honest medical products] must be distributed under protected labels”Thus was created the “Specific Medicines” Brand Name: Of which the formulation/manufacturing standards were set forth, right from the beginning by Dr. John Scudder [B] (1849-1894), is as follows:
1. First. . . the crude vegetable material [the flowering tops of the Female Hemp/cannabis plant], obtained at its proper season, is picked and dried.
[NOTE THE FOLLOWING:]
- "Obtained at its proper season” -- In our case the flowering tops of the female plant would be picked just before pollination.
- “Freshly picked” -- These words [not quoted above] appear in some of the descriptions authored by John Scudder. However, some exceptions are called for whenever freshly picked plant matter is not a practicality. NOTE: that as no physiological testing [on dogs] of any kind took place, the above processes was of critical importance. As was selecting plants that were strong in the active medical material in the first place.
-
IMPORTANT: This initial amount of plant matter (being about twice the eight ounces used by others), plus the selection process, guaranteed that it would be much stronger in strength than all others (including those manufactured as per USP standards) at the time. This explains why recommended doses for “Specific Medicine” tinctures were lower than for others.
Note here that the actual percolation process was done via a cold still apparatus, not a direct heating process like a coffee maker. [See patent No. 777,115 (Addendum-J) for percolator diagrams and details] Additionally, the exact amount of Cannabis resinous oil extracted by the process, will vary, depending upon the quality of the plant matter in question.
The Pure Food and Drug Act of 1906, required the label to state the exact percentage amount of Alcohol which varies [according to various labels that we have been able to locate] between 74% and 82% by volume --- the rest of the product is the extracted resinous oil of Cannabis. This is normal, think of it this way; every orange varies in the amount of vitamin C it has, even if the oranges come from the same tree.
Once in liquid form, "Tincture Cannabis" could then, easily, be prescribed/dispensed by the physician (so many drops in half a glass of warm water) etc. Note that the formulation of this product remained solid (and true to John Scudder’s formulation) throughout its entire life cycle --- from 1870, when it first appeared on the market, until 1945 (well after June 25, 1938) when the product was taken off the market.
CANNABIS TINCTURE (Specific Medicine) BY LLOYD BROTHERS Co.
FACTORS DEALING WITH LABELS:
Addendum-L - shows copies of various labels from 1870 -to the- 1940's for (Specific Medicines) Cannabis Tincture. Note that the labels for this product all seem to follow the changes in ownership of the Company itself. This company had the exclusive use of the (copy-protected) trade name... “Specific Medicines.”
REFERENCING VARIOUS LABELS [see Addendum-L]:
- A copy of the original (from product creation) label used by “H.M. Merrell & Co.,” as well as, the “Merrell, Thorp & Lloyd” corporation before 1883.
- A “Thorp & Lloyd Brothers” label [Era 1884-1885]. While it is not a picture of the Cannabis tincture in question, it does accurately represent EXACTLY what it would have looked like. Just replace the word Aconite with the word Cannabis. The formulation of ALL “Specific Medicines” [TM] was well known at that time.
- Both labels [Era 1886-1906] came into use between 1886 (when the firm became known under that name) and Jan 1, 1907, when the pure food and drug act of 1906 went into effect. Note that no mention of alcohol is made.
- A copy of the last label used between Jan 1, 1907 and early 1940's, when the product (mostly due to the Marihuana Tax Act) was taken off the market. Note that the label now, makes reference to its Alcohol content, as required by the pure food and drug act.
| DATE: | FIRM NAME: |
| 1870 | H.M. Merrell & Co. |
| 1877 | Merrell, Thorp, and Lloyd |
| 1884 | Thorp & Lloyd Brothers |
| 1885 | Lloyd Brothers Co. |
| 1924 | Lloyd Brothers Pharmacist, Inc |
BULK CANNABIS:
Documentation --- Establishing the eligibility of Bulk Cannabis (in all forms, e.g., Pressed, Loose Leaf, Sifted, Grounded, or Powdered forms), for Grandfathering: To prevent any and all confusion, Addendum-F, provides a definition of Bulk H.G.C. Cannabis.
PROOF OF MARKETING TIME LINE
[Jan 1, 1907 -thru- Sept, 1941] for BULK (H.G.C.) CANNABIS:
The following table is provided as proof that Bulk (H.G.C.) Medical Cannabis was being sold and marketed in the U.S. before Jan 1, 1907, and after June 25, 1938. It consists of a listing of various pharmaceutical trade magazines, purchasing records and price & product catalogs.
Marketing Time Line for Bulk Cannabis - 1901 -to-1941
|
Date:
|
Source:
|
Page:
|
Product:
|
| 1941 (Sept) | American Druggist Blue Book | pg. 347 | Powder Extract Cannabis |
| 1940 (June) | American Druggist Blue Book | pg. 59 | Powder Extract Cannabis |
| 1939 (June) | American Druggist Blue Book | pg. 51 | Powder Extract Cannabis |
| 1938 (June) | American Druggist Blue Book | pg. 60 | Powder Extract Cannabis |
| 1937 (July) | American Druggist Blue Book | pg. 208 | Powder Extract Cannabis |
| 1936 | Eli Lilly & Co. (PPC) | pg. 61 | Powder Ext. Cannabis |
| 1935 | J.L. Hopkins & Co. | pg. 5 | Granulated Cannabis |
| 1934 | Allaire Woodward (PPC) | pg. 6 | Powdered Extract Cannabis |
| 1933 (Nov) | Druggist Circular Red Book | pg. 21 | Cannabis USP |
| 1931 | R. Hillier's Son Co. (PPC) | pg. 14 | Powd. Ext. Cannabis |
| 1930 (Nov) | Druggist Circular Red Book) | pg. 24 | Cannabis U.S.P. |
| 1929 (Nov) | Druggist Circular Red Book) | pg. 12 | Herb - Cannabis Indica |
| 1928 (April) | Druggist Circular Red Book) | pg. | |
| 1927 | S.B. Penik & Co. (PPC) | pg. 11 | Powd. Ext. Cannabis |
| 1926 (Nov) | Druggist Circular Red Book | pg. 12 | Herb - Cannabis Indica |
| 1925 | Eli Lilly & Co. (PPC) | pg. 65 | Powd. Ext. Cannabis |
| 1924 | P.E.Anderson & Co. | pg. 11 | Powd. Ext. Cannabis |
| 1921 | H.K. Mulford (PPC) | pg. 11 | Powd. Ext. Cannabis |
| 1920 | Eimer & Amend (PPC) | pg. 79 | Powd. Ext. Cannabis |
| 1919 | Eli Lilly & Co. (PPC) | pg. 71 | Powd. Ext. Cannabis |
| 1918 | McKesson & Robbins (PPC) | pg. 37 | Powd. Ext. Cannabis |
| 1917 | Oil Paint & Drug Reporter | pg. 75 | Cannabis Ad |
| 1916 | Purchase Record - Eli Lilly | pg. | Bulk Cannabis |
| 1915 | Frederick stearns & Co. (PPC) | pg. 59 | Powd. Ext. Cannabis |
| 1914 (April) | Druggist Circular Red Book | pg. 12 | Powd ext. Cannabis Indica |
| 1913 | Parke Davis (PPC) | pg. 45 | Powd ext. Cannabis Indica |
| 1912 (Nov) | Druggist Circular Red Book | pg. 11 | Powd. Ext. Cannabis |
| 1911 | Purchase Record - Eli Lilly | pg. | Bulk Cannabis |
| 1910 | Parke Davis (PPC) | pg. 356 | Powd. Ext. Cannabis |
| 1909 | Hance Brothers & White | Pg3/343 | Powd. Ext. Cannabis |
| 1908 | New York Alkaloid (PPC) | pg. 4 | Powd. Ext. Cannabis |
| 1907 | Purchase Record - Eli Lilly | pg. | Bulk Cannabis |
| 1906 | E.L. Patch Co. (PPC) | pg. 88 | Powd. Indian Hemp |
| 1905 | Lloyd Brothers (PPC) | pg. 35 | Powd ext. Cannabis Indica |
| 1904 | Schieffelin & Co. (PPC) | pg. 14 | Ext Powder Cannabis Indica |
| 1902 | Henry Wampole (PPC) | pg. 52 | Powdered Cannabis |
| 1901 | Druggist Cooperative Co. (PPC) | pg. 26 | Powd. Ext. Cannabis |
-
TABLE CODE #:
PPC = Price and Product Catalog, from the stated Pharmaceutical Manufacturer. [selected pages can be found on enclosed CD-rom]
American Druggist Blue Book [a yearly Price and Product supplement, commonly referred to as simply; “The Blue Book.” ], issued yearly (July) as part of the American Druggist. A trade magazine to the Pharmaceutical industry.
Druggists Circular Red Book [ a bi-yearly Price and Product supplement, commonly referred to as simply; “The Red Book.”], issued twice a year (May, Nov) as part of the Druggist Circular. A trade magazine to the pharmaceutical industry.
Purchase Record - Eli Lilly = makes references to internal purchasing records from the Eli Lilly Co.
- Addendum - O, documents that Medical Cannabis is still being grown and marketed for medical purposes today, and thus has never physically been taken off market.
CONTROLLED SUBSTANCES & LABELING ISSUES:
Because our petition is so similar to one introduced by Paul Klopper and Dr. Tod Mikuriya, M.D. some years back [See Addendum-A], which was rejected. See FDA Rejection Letter Addendum - B. This is a good time to address one of the main reasons given [over labeling issues] for your original rejection of that Petition.
The FDA original rejection letter stated as follows:
-
“FDA notes that marihuana is currently listed in Schedule 1 under the Controlled Substances Act (21 U.S.C. 812(c). 21 CFR 1308.11(d)(19). The labeling for all Schedule 1 drugs is required to bear the “C-1” symbol (21 CFR 1302.03). FDA would regard the inclusion of the ”C-1” symbol on a product as a labeling change regarding the conditions of its use. This would be true even if marihuana were rescheduled and placed in Schedules 2 through 5: the inclusion of the “C” symbol on the product would be viewed as a labeling change regarding the conditions of its use.“ [Dennis E. Baker - Associate Commissioner for Regulatory Affairs]
- [A]- Due to the passage of the Controlled Substances Act (~1970), which now classifies Cannabis as a controlled substance, that -
- [B]- This act now requires the label (of any Cannabis product) to bare a “CI” (or CII, CIII, CIV, CV), corresponding to its control level, sign or symbol on the label, and that -
- [C]- Therefore (in his opinion), this OF-AND-BY itself, would be seen as a MAJOR label change and -
- [D]- Thus the FDA cannot approve the Grandfather petition.
With Reference to Addendum-C, in general, we are mystified by Mr. Bakers’ personal opinions on the matter. How it is possible for anyone to come to such conclusions, especially given the number of “Controlled Substances” which have already received FDA grandfather clause approval, is beyond logic, actual usage, and belief. Specific examples of other “C” label products follow:
- 1 - COCAINE (which is still manufactured/processed) for its medical properties in the U.S., is in fact BOTH a Controlled Substance (now subject to the same labeling issues that Cannabis would be) as well as a Grandfathered Drug.
- 2 - CODEINE PHOSPHATE (an opium derivative) which is used extensively in cough syrups today, is also BOTH a Controlled Substance (now subject to the same labeling issues that Cannabis would be) as well as Grandfathered Drug.
- 3 - CODEINE SULFATE (an opium derivative) which is used extensively in cough syrups today, is also BOTH a Controlled Substance (now subject to the same labeling issues that Cannabis would be) as well as Grandfathered Drug.
- 4 - OPIUM is also BOTH a Controlled Substance (now subject to the same labeling issues that Cannabis would be) as well as Grandfathered Drug.
- 5 - MORPHINE being re-FDA approved in 1980, still; it must be assumed that it had, between the years 1938 and 1980, been Grandfathered, despite being a Controlled Substance.
===========
Next, the petitioners mention the fact that once given FDA grandfather clause approval, NO ONE knows exactly what schedule (if any) Medical Cannabis will fall into.
As brought out by Mr. Baker, [21-USC-812], at present there are five established schedules of controlled substances; to be known as schedules I, II, III, IV, and V. Let us look at each one of them; including schedule VI (meaning non-control)
-
(1) Schedule I.
(A) The drug or other substance has a high potential for abuse.
(B) The drug or other substance has no currently accepted medical use in treatment in the United States.
(C) There is a lack of accepted safety for use of the drug or other substance under medical supervision.
-
(2) Schedule II.
(A) The drug or other substance has a high potential for abuse.
(B) The drug or other substance has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions.
(C) Abuse of the drug or other substances may lead to severe psychological or physical dependence.
-
(3) Schedule III.
(A) The drug or other substance has a potential for abuse less than the drugs or other substances in schedules I and II.
(B) The drug or other substance has a currently accepted medical use for treatment in the United States.
(C) Abuse of the drug or other substance may lead to moderate or low physical dependence or high psychological dependence.
-
(4) Schedule IV.
(A) The drug or other substance has a low potential for abuse relative to the drugs or other substances in schedule III.
(B) The drug or other substance has a currently accepted medical use for treatment in the United States.
(C) Abuse of the drug or other substance may lead to limited physical dependence or psychological dependence, relative to the drugs or other substances in schedule III.
(5) Schedule V.
(A) The drug or other substance has a low potential for abuse relative to the drugs or other substances in schedule IV.
(B) The drug or other substance has a currently accepted medical use in treatment in the United States.
(C) Abuse of the drug or other substance may lead to limited physical dependence or psychological dependence relative to the drugs or other substances in schedule IV.
But whether or not that point is accepted by the FDA is irrelevant, what the petitioners are noting at this time is that upon FDA approval, there is a good chance that the labeling issue will not even come up. And, in any case; for purposes of this petition, is NOT a relevant issue at this time. After and only after FDA grandfather approval is given, will the issue (probably in the courts) of exactly what control substance scheduling (if any) Cannabis will now fall under, will it ever come up ---- Not before or during grandfathering. Thus the labeling issue (as brought forth by Mr. Baker) of and by itself has NOTHING to do with the FDA grandfather clause approval process. We ask that the FDA simply do their job and enforce the law as written, and insure that cancer victims have full access to Medical Cannabis, and not create imaginary (a.k.a. political) barriers.
RELEVANT STATUTORY, REGULATORY, and JUDICIAL DECISIONS
The Administrator for the Drug Enforcement Agency has recognized that formulations prepared from Cannabis were marketed as medicine prior to 1938:
"Cannabis sativa L. was one of the first plants to be used by man for fiber, food, medicine, and in social and religious rituals. There were approximately 20 traditional medicinal uses of cannabis ... in Western medicine from the mid-19th to the early 20th century ... In 1941, marijuana passed out of the National Formulary and the United States Pharmacopoeia."54 Fed.Reg. 53767, 53774 (1989).
The Controlled Substance Act, 21 U.S.C. § 801 et seq., currently lists "marihuana" as a schedule I substance. See 21 U.S.C. § 812(I)(c)(10). Petitioner contends Congress did not "un-grandfather" the above listed products when it decided to place "marihuana" (generally) into the schedule I category. At the beginning of the statute setting forth the list of schedule I substances, Congress declared its intent to recognize previously grandfathered substances: "Unless specifically excepted ... any material, mixture, or preparation, which contains any quantity of the following ... (10) Marihuana." 21 U.S.C. § 812(I)(c).
The "unless specifically excepted" clause must be read to refer to 21 U.S.C. § 321(p) which "excepted" and accepted as medicine those products marketed prior to 1938. If Congress had intended to repeal marijuana's pre-1938 exemption as cannabis medicine under § 321(p), it would have made clear its intent to repeal that exemption. Tennessee Valley Authority v. Hill, 437 U.S. 153, 189-90 (1978) ("intention of the legislature to repeal must be clear and manifest.").
In Rutherford v. United States, 542 F.2d 1137, 1142n4. (10th Cir.1976), the court notes that a pre-1938 product could be un- grandfathered, but only when that previously grandfathered drug is found to be "dangerous to health." To date, neither Congress, the FDA, the DEA, nor the recently commissioned panel from the Institute of Medicine (see Marijuana and Medicine: Assessing the Science Base, 1999) have declared cannabis/marijuana "dangerous to health."
Since the present decision as to what is or what is not medicine rests with the FDA, (and ultimately the courts) the Controlled Substances Act (§ 801 et seq.) did not transfer or otherwise diminish the FDA's authority and responsibility to determine whether a product is a "new" or "exempt" drug or medicine under the grandfather clause:
"Clearly, the Controlled Substances Act does not authorize the Attorney General, nor by delegation the DEA Administrator, to make the ultimate medical and policy decision as to whether a drug should be used as medicine." ... "The FDA has both the experts and the statutory mandate to resolve conflicts over safety and efficacy of new drugs."57 Fed.Reg. 10499, 10505 (1992)
HEARING REQUESTED/REQUIRED PRIOR TO ANY ADVERSE RULING
As noted above, and as more fully set forth in the attachments, there are genuine and substantial issues of fact regarding the exempt status of the products listed in this petition. As such, a full hearing is required prior to any adverse ruling on the issues contained within this petition. See 21 CFR § 12.87(a). "The objective of a formal evidentiary hearing is the fair determination of relevant facts consistent with the right of all interested persons to participate and the public interest in promptly settling controversial matters affecting the public health and welfare."
In controversial matters affecting the public health and welfare, the Commissioner of the Food and Drug Administration is required to produce a "full administrative record" which includes a "full hearing" to give "proponents an opportunity to express their views." Rutherford, 542 F.2d. at 1143; accord Breitmeyer v. Califano, 463 F.Supp. 810, 815 (E.D.Mich 1978) ("Under 21 CFR § 314.200(d), any interested person may request a hearing. The hearing, once granted, would extend to all issues relating to [the product's] status as a new drug, including exemption under the grandfather clause. 21 CFR § 314.200(e)(2).").
CERTIFICATION and VERIFICATION
The undersigned certify, that, to the best knowledge and belief of the undersigned, this petition and notice of exemption includes all information and views on which the petition relies, and that it includes representative data and information known to the petitioners which are both favorable and unfavorable to the petition.
The undersigned verify that all appropriate records have been searched and to the best of their knowledge and belief it includes a true and accurate presentation of the documents and facts.
Signed:
|
_____________________________ |
Dated: April, ____, 2010 |
Mikuriya Medical
PO Box 9143
Berkeley, CA 94703
|
_____________________________ |
Dated: April, ____, 2010 |
36360 Peterson Rd.
Auberry, Ca. 93602
FOOTNOTES:
[A] - The Eclectic Medical Movement, can best be thought of as a rival to the AMA’s allopathic movement.
[B]- Dr. John Scudder was another major figure with the Eclectic Medical Movement.
WANT TO KNOW MORE:
=====================
Due to space / download time considerations, only selected materials are displayed. If you would like to obtain more information, feel free to contact the museum. All our material is available (at cost) on CD-Rom format.
CONTACT PAGE
 BACK TO PETITION INDEX |
FDA PETITION  On to the First Addendum |